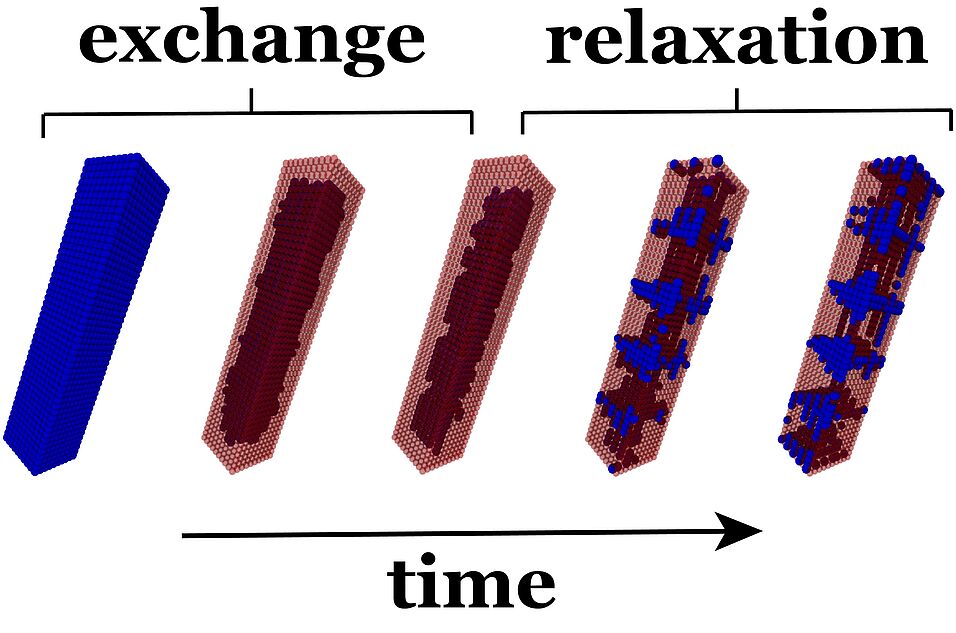
Cation exchange - the replacement of one species of ions with another - is an efficient and common method to modify the composition of nanocrystals post-synthesis. Despite its practical importance, the microscopic mechanism that governs this nonequilibrium process, has remained largely unclear. Now, the research team from Vienna and Berkeley has studied cation exchange in a simple lattice model that captures the key features of the process: the differing time scale of surface exchange and internal diffusion, and the lattice mismatch caused by the different sizes of the exchanging ions. Using kinetic Monte Carlo simulations, the scientists demonstrate that these two factors explain the nonequilibrium patterns observed in cation exchange experiments and they show that elastic interactions are at the origin of this behavior. These results help to understand the heterostructures produced in nanocrystals by cation exchange and suggest strategies for the design of patterns in nanoscale materials.
Publication:
Layne B. Frechette, Christoph Dellago and Phillip L. Geissler, “Elastic forces drive nonequilibrium pattern formation in a model of nanocrystal ion exchange“, in Proceedings of the National Academy of Sciences of the USA 118, e2114551118 (2021) ; DOI: 10.1073/pnas.2114551118
Contact
Univ.-Prof. Dr. Christoph Dellago
Computational and Soft Matter Physics
Faculty of Physics
1090 - Wien, Kolingasse 14-16
+43-1-4277-51260
christoph.dellago@univie.ac.at