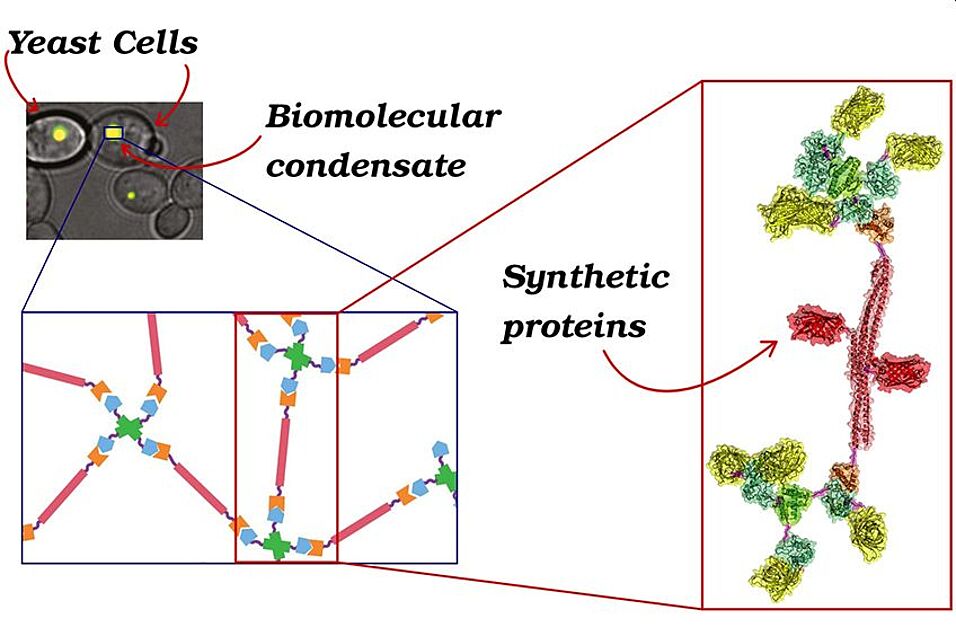
The international team - featuring a prominent contribution from the University of Vienna - has developed a synthetic system able to pave the way to a deeper comprehension of the cellular working principles. Such work could generate positive effects in different scientific fields.
Indeed, cells are the building blocks of any living being; their optimal state is based on a precise organization of the internal space, such that proteins and nucleic acids are allowed to efficiently function. Until a decade ago, it was believed such organization was uniquely due to the presence of organelles, secluded from the rest of the cytoplasm by membranes. However, recently it has been discovered that a certain number of organelles, also named biomolecular condensates, lack such a membrane; they carry out an important role in the cellular homeostasis, being able to react to chemical variations inside and outside the cell. Moreover, the mechanisms governing the formation of these organelles, mainly made of proteins and nucleic acids, are believed to be involved in the pathogenesis of diseases such as Alzheimer's, ALS and frontotemporal dementia, syndromes caused by an anomalous aggregation of proteins. Unfortunately, the vast number of components in the cytoplasm makes it difficult to identify which mechanism determine the formation and dissolution of these condensates in physiological conditions.
In order to overcome this problem, researchers from the Weizmann Institute of Tel Aviv and from the Universities of Oxford, Vienna and Rome have genetically engineered yeast cells in order to produce proteins able to form biomolecular condensates with controllable physicochemical properties.
"Thanks to the use of state-of-the-art experimental methods and to the comparison with theoretical and numerical models - says Emanuele Locatelli, from the Faculty of Physics of the University of Vienna - we have been able to understand how the microscopic properties of proteins and their concentration influence the formation and the features of biomolecular condensates".
The results, published in the prestigious journal Nature Chemical Biology, represent a step forward to understanding which physicochemical properties of the proteins majorly influence the role, both physiological and pathological, of the condensates.
"The system developed is very general - adds Emanuele Locatelli - and can be used as well to investigate questions of great biological relevance concerning microscopic processes, happening in cells and not observable otherwise, e.g. the specific interactions between proteins and nucleic acids." Thus, this represent a step forward towards a more profound comprehension of the mechanisms through which biomolecular aggregation can influence the behavior of cells.
Publication in Nature Chemical Biology
Wissenschaftlicher Kontakt
Univ.-Prof. Dipl.-Ing. Dr. Christos N. Likos
Fakultät für Physik
Universität Wien
1090 - Wien, Sensengasse 8/15
+43-1-4277-732 30
christos.likos@univie.ac.at