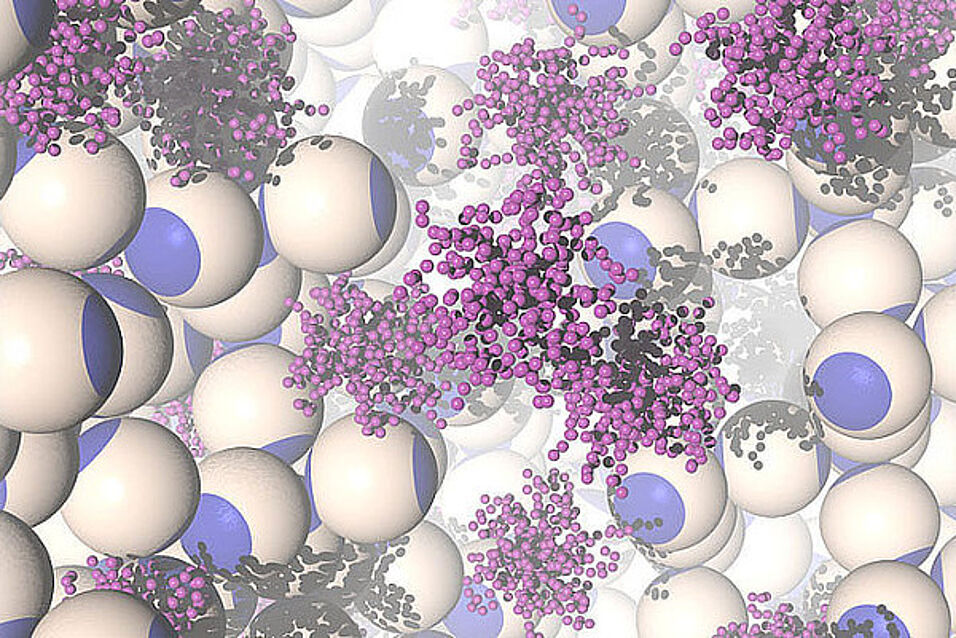
Crystals are solid materials composed of microscopic building blocks arranged in highly ordered patterns. They have countless applications, ranging from metallurgy to jewellery to electronics. Many of the properties that make crystals useful depend on the detailed pattern of arrangement of their constituents, which, in turn, is highly sensitive to the details of the interaction between the building blocks. In molecular and atomic crystals the interparticle forces are fixed by Nature, and the only way of tuning the microscopic arrangement is to either vary the external conditions (temperature, pressure, etc.) or change the particles themselves. By contrast, in soft matter Physics, where the building blocks are orders of magnitude larger and much more complex than atoms, it is possible to design and engineer building blocks with extremely tunable properties. Consequently, much effort has been devoted to the synthesis of colloids that self-assemble into highly symmetric patterns with technologically relevant properties. For instance, there exist specific crystal lattices that exhibit very exciting optical properties, the so-called photonic crystals – periodic structures that allow certain bands of
wavelengths of light to propagate through
their interior while blocking other ones.
A natural example of a photonic crystal is the opal, whose fascinating coloration is due to the way the light interacts with its microscopic structure of colloidal particles arranged on a regular lattice. The multicolored iridescence of the precious opal, the source of its charming appearance, is due to the presence of several small crystals, known as crystallites, which are randomly oriented with respect to one another. In addition, the assembly of colloidal crystals is often confounded by polymorphism: "Different structures are characterised by comparable thermodynamic stabilities, making it difficult to produce a single morphology at will", says Christos Likos from the Faculty of Physics of the University of Vienna.
The resulting lack of long-range order is detrimental for many applications. Accordingly, strategies need to be developed that enhance the growth of long-ranged, monocrystalline samples in (real or numerical) experiments. Accordingly, scientists have been working hard to deveop strategies that enhance the growth of large, monocrystaline structures. Employing computer simulations, a new method has now been deveoped that allows the assembly of technologically relevant, non-polymorphic crystals. "The system crystallises into a mixture of difference micocrystals. However, the competing structures assembled by the colloids have different geometries and different internal void distributions. This difference can be exploited by tuning the size of polymer additive to interact uniquely with the void symmetry of the desired crystal, effectively stabilising it against the competitor", explains Lise-Meitner Fellow Lorenzo Rovigatti, working at the group of Christos Likos.
The results of the research team serve not only to illustrate an alternative to existing approaches which, in many cases, produce unsatisfactory results, but also to guide experimental realizations of highly-ordered colloidal open crystals in the near future.
Publication in "ACS-Nano"
Nathan A. Mahynski, Lorenzo Rovigatti, Christos N. Likos, and Athanassios Z. Panagiotopoulos
DOI 10.1021/acsnano.6b01854
The project was supported by the Austrian Science Fund (FWF) through the Lise-Meitner Fellowship M 1650-N27.